Website owner: James Miller
Types of chemical reactions: Exothermic, endothermic, combination, decomposition, single replacement, double replacement, combustion, acid-base, oxidation-reduction, hydrolysis. Heat of formation. Chemical equilibrium. Law of mass action.
Chemical reactions and energy changes. When a chemical reaction takes place, an energy change is always involved. Energy is either released or it is absorbed during the reaction. This energy that is released or absorbed is generally in the form of heat, light or electricity. When we burn wood, carbon unites with oxygen in the reaction
C + O2 → CO2
and both heat and light are produced. The chemical reactions in an automobile storage battery produce electricity. Many other chemical reactions require an input of heat, light or electricity in order for them to occur. In the electrolysis of water electrical energy is required to make the H2O molecules decompose into hydrogen and oxygen gas.
Def. Exothermic reaction. A reaction which releases (i.e. produces) energy as it takes place.
Def. Endothermic reaction. A reaction which requires energy in order for it to take place.
Types of chemical reactions. Although there are many ways of classifying chemical reactions, and no scheme is perfect, the following types of chemical reactions are generally recognized:
1. Combination (or synthesis)
2. Decomposition
3. Single replacement (or substitution or displacement)
4. Double replacement (or double displacement or metathesis)
5. Combustion
6. Acid-base
7. Oxidation-reduction (or redox)
8. Isomerization
9. Hydrolysis
1. Combination (or synthesis) reactions. A combination (or synthesis) reaction is one in which two or more elements or compounds combine to form a single product. A general equation for this type of reaction is
A + B → AB
where A stands for one element, B stands for another element, and AB is the compound formed.
Examples.
2Na (s) + Cl2 (g) → 2NaCl (s)
MgO (s) + H2O (l) → Mg(OH)2 (aq)
SO2 (g) + H2O (l) → H2SO3 (aq)
Most combination reactions release energy i.e. they are exothermic reactions.

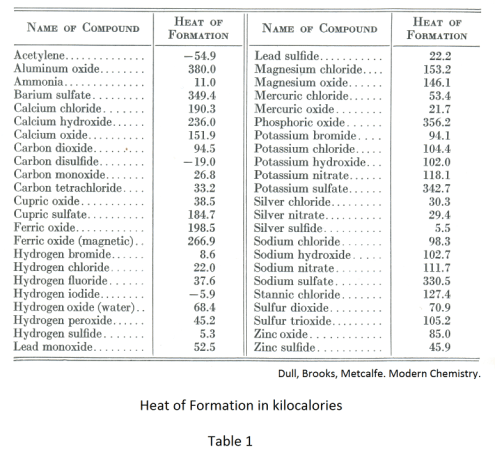
Def. Heat of formation. The heat given out (or taken in) during the formation of one mole of a compound from its constituent elements under standard conditions of temperature and pressure.
Table 1 shows the heat of formation of some common substances. When heat is liberated, as in exothermic reactions, the heat of formation is positive. When heat is absorbed, as in endothermic reactions, the heat of formation is negative.
One can see from the table that when one mole of carbon dioxide is formed from carbon and oxygen, 94.5 kilocalories of heat are given off. Inspection of the table shows that the vast majority of the numbers are positive, which means heat is given off in the formation of the compound. Only a few compounds such as hydrogen iodide and carbon disulfide require energy for their formation.
The amount of energy required to decompose any compound back into its constituent elements is exactly the same amount as is given off when it is formed. For example, the amount of heat required to decompose a mole water (H2O) into hydrogen and oxygen is 68,400 calories, the same amount that is given off when it is formed.
Stability of compounds. Compounds with high heats of formation are stable since it takes a great deal of energy to decompose them. Those with low heat of formation are relatively unstable since only a small amount of energy is required to decompose them. Compounds with a negative heat of formation are not only unstable but may be explosive.
The reaction in which a compound with a high heat of formation is formed is spontaneous once it has started and generally very vigorous. An example is the burning of wood or coal. Once started, the reaction generally proceeds vigorously. Reactions for compounds with lower heats of formation are less vigorous and such compounds are rather easily decomposed. An example: mercuric oxide with a heat of formation of 21,800 calories. It is formed at a temperature of 300o C. With stronger heating, however, it decomposes into its elements again.
A compound with a negative heat of formation requires energy to produce it. It decomposes easily and will probably decompose spontaneously. An example is carbon disulfide with a negative heat of formation of -19,000 calories. It is only formed at red heat and its instability is shown by the fact that it is very flammable.
Predicting the product of a composition reaction. The product of a composition reaction will obviously be some combination of the reacting elements, however, if one of the reacting elements is a variable valence element, it may be difficult to predict which valence value the element will assume.
2. Decomposition reactions. Decomposition reactions are those in which a compound is decomposed by heat, light, or electricity into simpler compounds or into elements. The general equation for this type reaction is
AB → A + B
where AB is the original compound and A and B are elements or simpler compounds into which the original compound decomposes.
Example.
2 H2O → 2 H2 + O2 (electrolysis of water)
Six types of decomposition reactions. There are six types of decomposition reactions:
1] Metallic carbonates, when heated, form metallic oxides and carbon dioxide.
Example. Limestone, CaCO3, on being heated, will form lime, CaO, with carbon dioxide given off as a gas.
CaCO3
![]() CaO + CO2↑
CaO + CO2↑
2] Most metallic hydroxides, when heated, decompose into metallic oxides and water.
Example. If slaked lime Ca(OH)2 is heated strongly, steam is given off and quicklime, CaO, remains.
Ca(OH)2
![]() CaO + H2O↑
CaO + H2O↑
3] Metallic chlorates, when heated, decompose into nonmetallic oxides and water.
Example. 2 KClO3
![]() 2 KCl + 3 O2
2 KCl + 3 O2
4] Some acids, when heated, decompose into nonmetallic oxides and water.
Examples.
H2CO3
![]() H2O + CO2↑
H2O + CO2↑
H2SO3
![]() H2O + SO2↑
H2O + SO2↑
H2SO4
![]() H2O + SO3↑
H2O + SO3↑
5] Some oxides, when heated, decompose.
Most oxides are very stable compounds and there are only a few which will decompose on heating. Two are the following:
Examples.
2 HgO
![]() 2 Hg + O2↑
2 Hg + O2↑
2 PbO2
![]() 2 PbO + O2↑
2 PbO + O2↑
6] Some decomposition reactions are produced by electricity.
Examples.
2H2O
![]() 2H2↑ + O2↑
2H2↑ + O2↑
2 NaCl + 2H2O
![]() H2↑ + Cl2↑+ 2NaOH
H2↑ + Cl2↑+ 2NaOH
3. Single replacement (or substitution or displacement) reactions. A single replacement (or displacement) reaction is characterized by one element being displaced by another element in a compound (i.e. a more active element displaces a less active element in a compound). These reactions have the general form
A + BC → AC + B
where A is a metal which displaces the positive part of the compound BC to form AC and liberate B or the form
D + EF → ED + F
where D is a nonmetal which displaces the negative part of the compound EF to form ED and liberate F.

Example.
Zn + 2 HCl → ZnCl2 + H2
Compounds formed by displacement have higher positive heats of formation than the original compounds. Evolution of energy causes the reactions to take place. The quantities of heat liberated in single replacement reactions are generally less than in combination or decomposition reactions. Chemists use the activity series to help predict the course of single replacement reactions. See Table 2. This table separately lists the metallic and nonmetallic elements in order of their activity i.e. they are listed in the order of their ability to displace one another in compounds, where those higher on the list will displace those lower on the list. Thus, on the metals list, potassium will replace sodium or zinc and on the nonmetals list fluorine will replace, say, bromine.
Four types of single replacement reactions. There are four types of single replacement reactions.
1] Replacement of a metal in a compound by a more active metal.
Example. Fe + CuSO4 → FeSO4 + Cu↓
We note that iron, Fe, is higher than copper, Cu, on the activity series list.
2] Replacement of hydrogen in water by active metals.
Theoretically all metals above hydrogen in the activities series should replace it. However, temperature is an important factor. Potassium, sodium, and calcium replace hydrogen in water at ordinary temperatures. Magnesium will replace it in hot water. Red-hot iron will replace it from steam.
Example. 2 Na (s) + 2 H2O (l) → 2 NaOH (aq) + H2 (g)
Note. It is important to distinguish between the displacement of hydrogen from an acid and displacement of hydrogen from water. Sodium is highly active and is able to displace hydrogen from water but less active metals like zinc cannot displace hydrogen from water but do readily replace it from acids:
Zn (s) + H2SO4 (aq) → ZnSO4 (aq) + H2 (g)
3] Replacement of hydrogen in acids by active metals.
Example. Zn + 2 HCl → ZnCl2 + H2
4] Replacement of halogens.
The halogens are fluorine, chlorine, bromine and iodine. Any one will replace any below it in the activities series.
Example. 2 KI + Cl2 → 2 KCl + I2
Chemical equilibrium. Many chemical reactions are reversible and they proceed in one direction or the other until equilibrium is established. Chemical equilibrium exists when the rate of the reaction in the forward direction equals the rate of the reaction in the reverse direction. For example, consider the reaction
N2 + 3 H2 ⇆ 2NH3
taking place in a sealed reaction chamber. Here nitrogen and hydrogen gases react to form gaseous ammonia, NH3 . When nitrogen and hydrogen are first introduced into the reaction chamber, they begin to form ammonia molecules. As the concentration of ammonia increases, ammonia molecules start to decompose, forming nitrogen and hydrogen. When the rate at which ammonia is formed equals the rate at which it decomposes, the system is at equilibrium. A reaction at equilibrium never goes completely to completion; molecules of reactants continue to collide to form product molecules, and product molecules constantly decompose to form reactant molecules.
Law of mass action. The rate of a chemical reaction is directly proportional to the molar concentrations of the reacting substances.
In the law of mass action concentrations are usually expressed in moles per liter and the rate or speed of a chemical reaction is defined as the quantity reacting per unit time.
Consider the following reversible reaction in a homogeneous solution:
a1A1 + a2A2 + ... anAn ⇆ b1B1 + b2B2 + ... + bmBm
Let [A1] = the concentration of A1 in moles per liter of mixture
[A2] = the concentration of A2 in moles per liter of mixture
.........................................................................................
[B1] = the concentration of B1 in moles per liter of mixture
[B2] = the concentration of B2 in moles per liter of mixture
.........................................................................................
and
S+ = the speed of the reaction in the forward direction
S_ = the speed of the reaction in the reverse direction
Then the law of mass action can be stated mathematically as
S+ = k+ × [A1]a1 × [A2]a2 × ... × [An]an
S_ = k_ × [B1]b1 × [B2]b2 × ... × [Bn]bm
where k+ and k_ are proportionality constants (velocity constants) which are dependent in general on temperature but not on pressure or concentration.
Condition for equilibrium. Equilibrium is reached when S+ = S_. At equilibrium,
k+ × [A1]a1 × [A2]a2 × ... × [An]an = k_ × [B1]b1 × [B2]b2 × ... × [Bm]bm

The quantity Kc = k+ /k_ is the concentration equilibrium constant of the reversible reaction.
Problem. In the reaction
N2 + 3 H2 ⇆ 2 NH3
A1 = N2, a1 = 1, A2 = H2, a2 =3, B1 = NH3, b1 = 2
and
S+ = k+ × [N2] × [H2]3
S_ = k_ × [NH3]2
Kc = k+ /k_ = [NH3]2 /( [N2] × [H2]3 )
Methods of forcing reversible reactions to completion. Chemists employ various methods to force or drive a reversible reaction in the direction they wish it to go. We list some:
1] By a change in temperature.
Example. At a moderate temperature, mercuric oxide is formed by heating mercury in the presence of air or oxygen in accordance with the equation:
2 Hg + O2 (gentle heat) → 2HgO
This reaction can be reversed and driven in the opposite direction by heating the mercuric oxide strongly. The mercuric oxide then decomposes according to:
2 HgO (strong heat) → 2Hg + O2↑
2] By a change in pressure.
Example. In order to prepare hydrogen chloride, we heat a mixture of sodium chloride and sulfuric acid. The reaction normally runs to completion with the evolution of hydrogen chloride gas according to the equation
Na+ + Cl- + H+ + HSO4- → Na+ + HSO4- + HCl↑
We can, however, drive the reaction to the left by putting the hydrogen chloride gas under pressure and forcing it back into the solution where it ionizes
3] By a change in concentration. Let us suppose that the reversible reaction
A + B ⇆ C + D
has reached a state of equilibrium. Then it can be seen that doubling the concentration of A will increase the number of contacts with B and this will increase the speed of the reaction toward the right. Thus by the tactic of increasing the amount of one of the reagents, we can force a reaction in one direction or the other. We are utilizing the law of mass action to control the direction.
4. Double replacement (or double displacement or metathesis) reactions. Double replacement reactions are those in which two compounds exchange ions to produce two new compounds. These reactions have the general form
AB + CD → AD + CB
where A and C are the positive ions of compounds and B and D are the negative ions.
Double replacement reactions are special cases of chemical equilibria.
Examples.
NaCl (aq) + AgNO3 (aq) → NaNO3 (aq) + AgCl (s)
Pb(NO3)2 + 2 KI → PbI2 + 2 KNO3
Some chemical reactions run to completion and other do not. Chemists can predict that certain double replacement reactions will run to completion. What things may cause a double replacement reaction to go to completion? If a precipitate is formed, a gas is formed, or water is formed, this will remove some of the ions from the solution and cause the reaction to go to completion.
Example. If we mix solutions of sodium chloride and silver nitrate, a white precipitate of silver chloride immediately forms leaving Na+ and NO3- ions in the solution:
Na+ + Cl- + Ag+ + NO3- → AgCl↓ + Na+ + NO3-
5. Combustion reactions. A combustion reaction is a type of redox reaction in which a combustible material combines with an oxidizer to form oxidized products and generate heat (exothermic reaction). Usually in a combustion reaction oxygen combines with another compound to form carbon dioxide and water.
Example. The burning of naphthalene:
C10H8 + 12 O2 → 10 CO2 + 4 H2O
6. Acid-Base Reactions. An acid-base reaction is a type of double displacement reaction that occurs between an acid and a base. The H+ ion in the acid reacts with the OH- ion in the base to form water and an ionic salt. A general equation for this type of reaction is
HA + BOH → H2O + BA
Example. HBr + NaOH → NaBr + H2O
7. Oxidation-reduction (redox) reactions. An oxidation-reduction reaction is any chemical reaction in which the oxidation number (valence) of an atom changes by gaining or losing an electron.
Example. In the reaction
C + O2 → CO2↑
the oxidation number of C goes from 0 to 4 and the oxidation number of O goes from 0 to -2.
Single replacement reactions are all oxidation-reduction reactions. In each one, some element is oxidized and some element is reduced. Combustion reactions are all redox reactions. Some combination and decomposition reactions are redox reactions. Double replacement reactions sometimes involve oxidation and reduction, but not usually.
8. Isomerization reactions. In an isomerization reaction, the structural arrangement of a compound is changed but its net atomic composition remains the same.
9. Hydrolysis reactions. Hydrolysis is the reaction between a salt and water to yield an acid or a base.
Let us dissolve some sodium carbonate (Na2CO3) in water and test the solution with litmus paper. We find that it turns the litmus paper blue. The solution of this salt that we might expect to be neutral tests to be a base. It contains OH- ions. Why?
Let us dissolve some zinc chloride (ZnCl2) in water and test the solution with litmus paper. It turns the litmus paper red. The solution of this salt that we might expect to be neutral tests to be an acid. It contains H+ ions. Why?
The answer is that the OH- and H+ ions come from an ionization of the water. In the first case the Na+ ions of the Na2CO3 and the OH- ions of the water form the strong base sodium hydroxide (NaOH) and the H+ ions of the water and the CO3= ions form the weak acid H2CO3. A similar thing occurs on the second example. The Cl- ions of ZnCl2 and the H+ of the water form the strong acid HCl and the Zn+ ions of ZnCl2 and the OH- ions of the water form the weak base Zn(OH)2.
Na2CO3 → 2 Na+ + CO3=
2 H2O → 2 H+ + 2 OH-
2 Na+ + CO3= + 2 H+ + 2 OH- → 2 NaOH + H2CO3
ZnCl2 → Zn++ + 2 Cl-
2 H2O → 2 H+ + 2 OH-
Zn++ + 2 Cl- + 2 H+ + 2 OH- → Zn(OH)2 + 2 HCl
Rules:
1. Salts of weak acids and strong bases hydrolyze in water to form a base.
2. Salts of strong acids and weak bases hydrolyze in water to form an acid.
3. Salts of strong acids and strong bases do not hydrolyze in water.
4. Salts of very weak bases and very weak acids hydrolyze almost completely.
Example. Aluminum sulfide (Al2S3) is a salt of a weak acid and weak base. If we dissolve it in water we get the reaction
Al2S3 → 2 Al+++ + 3 S=
6 H2O → 6 H+ + 6 OH-
2 Al+++ + 3 S= + 6 H+ + 6 OH- → 2 Al(OH)3↓ + 3 H2S↑
where aluminum hydroxide is insoluble and precipitates out and hydrogen sulfide is volatile and escapes as a gas.
References
Dull, Brooks, Metcalfe. Modern Chemistry.
Schaum, Beckman, Rosenberg. College Chemistry. (Schaum)
Jesus Christ and His Teachings
Way of enlightenment, wisdom, and understanding
America, a corrupt, depraved, shameless country
On integrity and the lack of it
The test of a person's Christianity is what he is
Ninety five percent of the problems that most people have come from personal foolishness
Liberalism, socialism and the modern welfare state
The desire to harm, a motivation for conduct
On Self-sufficient Country Living, Homesteading
Topically Arranged Proverbs, Precepts, Quotations. Common Sayings. Poor Richard's Almanac.
Theory on the Formation of Character
People are like radio tuners --- they pick out and listen to one wavelength and ignore the rest
Cause of Character Traits --- According to Aristotle
We are what we eat --- living under the discipline of a diet
Avoiding problems and trouble in life
Role of habit in formation of character
Personal attributes of the true Christian
What determines a person's character?
Love of God and love of virtue are closely united
Intellectual disparities among people and the power in good habits
Tools of Satan. Tactics and Tricks used by the Devil.
The Natural Way -- The Unnatural Way
Wisdom, Reason and Virtue are closely related
Knowledge is one thing, wisdom is another
My views on Christianity in America
The most important thing in life is understanding
We are all examples --- for good or for bad
Television --- spiritual poison
The Prime Mover that decides "What We Are"
Where do our outlooks, attitudes and values come from?
Sin is serious business. The punishment for it is real. Hell is real.
Self-imposed discipline and regimentation
Achieving happiness in life --- a matter of the right strategies
Self-control, self-restraint, self-discipline basic to so much in life