Website owner: James Miller
Matter, Changes in matter, Structure of an Atom, Molecule
Matter. Anything which occupies space and has weight. Examples: Solids
such as wood, iron, copper, gold, and salt; Liquids such as water,
alcohol, gasoline and turpentine; Gases such as oxygen or acetylene.
Composition of Matter. Chemists have found that all complex substances
--- wood, steel, glass, plastics, even the waters of the ocean and the
air we breathe --- are mixtures of chemical compounds. Nearly a million
compounds have been identified, and these, in turn, are merely different
combinations of only about a hundred chemical elements known to science.
A compound is a substance consisting of a particular type of molecule.
A molecule consists of two or more atoms that are chemically bonded
together. Atoms are composed of neutrons, protons and electrons. There
are around a hundred kinds of atoms corresponding to the different
chemical elements.
Molecule. The smallest particle into which matter may be divided without
destroying its characteristic properties. It is a particle of matter
consisting of one or more atoms chemically bonded together.
Atom. The smallest particle of a chemical element.
Three Types of Changes in Matter.
1. Physical
2. Chemical
3. Nuclear
Physical Change. In a physical change the composition of the molecules of
the substance is not changed. Examples: water freezing, dissolving sugar
in water.
Chemical Change. In a chemical change the composition of the molecules of
the substance is changed, and new substances with new properties are
produced. Example: Iron rusting to produce iron oxide.
Nuclear Change. In a nuclear change new materials are formed by changes
in the identity of the atoms themselves. Example: gradual change of
radium atoms into lead atoms.

Structure of an atom. An atom is generally viewed as containing a central core called the nucleus about which particles called electrons revolve in a manner similar to the way a planet orbits the sun. The nucleus consists of several kinds of particles, the main ones being protons and neutrons. Protons carry a unit of positive charge, neutrons carry no charge, and electrons carry a unit of negative charge. All atoms have the same number of electrons as protons. Thus the atom as a whole is electrically neutral.
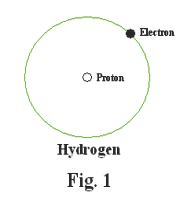
Let us now consider the structure of the simplest atom, the hydrogen atom. A hydrogen atom consists of a single electron orbiting a nucleus that consists of a single proton. See Fig. 1. The electron orbits the nucleus in what is called the K shell.
There are a couple of variations of the hydrogen atom called isotopes. In one isotope called Deuterion the nucleus contains a proton and a neutron. In yet another isotope, Tritium, the nucleus contains a proton and two neutrons. Except for that the atoms are identical. In general, the isotopes of a particular element differ by the number of neutrons in the nucleus.
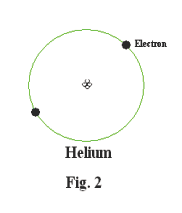
Let us now consider the next simplest element, helium. A helium atom consists two electrons orbiting a nucleus that consists of two protons and two neutrons. Both of these electrons orbit in the K shell. See Fig. 2.
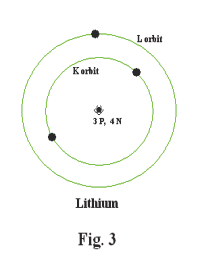
The next simplest element is Lithium. It has 3 protons and 4 neutrons in its nucleus and 3 electrons. Two of the electrons orbit in the K shell and one in a shell further out called the L shell. See Fig. 3.
We can progress in this way to progressively more complicated atoms. The number of protons in the nucleus of an atom is called its atomic number. It is the atomic number that distinguishes between elements, that identifies an element. There are over100 elements, each with its own atomic number. The atomic numbers run progressively 1, 2, 3, ... , 100+.
Almost the entire mass of an atom comes from its protons and neutrons. The electrons contribute almost nothing to the mass. An electron weighs about 1/1837 of a proton. Protons and neutrons weigh the same. There is a weight scale devised for atomic size weights. In this system the weight of a proton is one atomic weight unit (a.w.u.). Thus the weight of the hydrogen atom is 1 a.w.u.(we neglect the weight of the electron), the weight of a helium atom is 4 a.w.u., the weight of a lithium atom is 7 a.w.u., etc.
Size of an atom. A helium atom has a diameter of about 1 Angstrom (10-10 meters) while its nucleus has a diameter of only 1 femtometer (10-15 meters). Thus the diameter of the atom is 100,000 times that of the nucleus. If you made a scale model of an aluminum atom with a nucleus the size of a marble, the outermost electrons would fly around the nucleus at a distance of 150 feet. An atom is thus mostly empty space.
Atoms are extremely small, so small it is hard to imagine a particle so small. For example, if a single drop of water were magnified to the size of the earth, the individual atoms would be about the size of tennis balls.
Orbiting of the electrons. To visualize the movement of electrons about the nucleus envision an imaginary hollow sphere on which electrons move at enormous speeds in all directions, their orbital planes cutting through the sphere’s center in random directions. Occasionally the electrons may come close to each other and because of their similar charges they may veer apart before striking and continue in new directions, in new orbits. On more complex atoms there are several spheres, one inside another, on which they move.
Distribution of electrons in shells. There are a total of seven shells, labeled with letters from the alphabet: K, L, M, N, O, P, and Q. Below we give the electron distribution in the various shells for the elements with atomic numbers ranging from 1 to 56 plus a few others.
_____________________________________________________________________
Table 1
Shell
Element Atomic No. K L M N O P Q
Hydrogen 1 1
Helium 2 2
Lithium 3 2 1
Beryllium 4 2 2
Boron 5 2 3
Carbon 6 2 4
Nitrogen 7 2 5
Oxygen 8 2 6
Fluorine 9 2 7
Neon 10 2 8
Sodium 11 2 8 1
Magnesium 12 2 8 2
Aluminum 13 2 8 3
Silicon 14 2 8 4
Phosphorus 15 2 8 5
Sulfur 16 2 8 6
Chlorine 17 2 8 7
Argon 18 2 8 8
Potassium 19 2 8 8 1
Calcium 20 2 8 8 2
Scandium 21 2 8 9 2
Titanium 22 2 8 10 2
Vanadium 23 2 8 11 2
Chromium 24 2 8 13 1
Manganese 25 2 8 13 2
Iron 26 2 8 14 2
Cobalt 27 2 8 15 2
Nickel 28 2 8 16 2
Copper 29 2 8 18 1
Zinc 30 2 8 18 2
Gallium 31 2 8 18 3
Germanium 32 2 8 18 4
Arsenic 33 2 8 18 5
Selenium 34 2 8 18 6
Bromine 35 2 8 18 7
Krypton 36 2 8 18 8
Rubidium 37 2 8 18 8 1
Strontium 38 2 8 18 8 2
Yttrium 39 2 8 18 9 2
Zirconium 40 2 8 18 10 2
Niobium 41 2 8 18 12 1
Molybdenum 42 2 8 18 13 1
Technetium 43 2 8 18 14 1
Ruthenium 44 2 8 18 15 1
Rhodium 45 2 8 18 16 1
Palladium 46 2 8 18 18
Silver 47 2 8 18 18 1
Cadmium 48 2 8 18 18 2
Indium 49 2 8 18 18 3
Tin 50 2 8 18 18 4
Antimony 51 2 8 18 18 5
Tellurium 52 2 8 18 18 6
Iodine 53 2 8 18 18 7
Xenon 54 2 8 18 18 8
Cesium 55 2 8 18 18 8 1
Barium 56 2 8 18 18 8 2
....................................................................................................................................
Platinum 78 2 8 18 32 17 1
Gold 79 2 8 18 32 18 1
Mercury 80 2 8 18 32 18 2
Thallium 81 2 8 18 32 18 3
Lead 82 2 8 18 32 18 4
Bismuth 83 2 8 18 32 18 5
Radon 86 2 8 18 32 18 8
Radium 88 2 8 18 32 18 8 2
Uranium 92 2 8 18 32 21 9 2
_____________________________________________________________________
Examination of the above table will show how electrons are added to shells as one progresses to successively higher atomic numbers. First shell K fills to its maximum of two. Then shell L fills to its maximum of 8. Then shell M fills to 8 with Argon. Then shell N fills to 2 with Calcium. All goes regularly through Calcium. Then with Scandium, element 21, electrons start going into shell M again and it starts filling to a maximum of 18. Note the irregularity at Chromium where suddenly two electrons are added to the M shell and one is lost in the N shell. Note a similar phenomenon with Copper and others. Thus we see that the process is quite regular but with an occasional interesting irregularity.
The number of electrons in the outer shells is of special significance in determining how elements combine with each other in forming molecules. The capacity of an element for combining with another element or radical is given by a number called its valence. Following is a table of valences for various elements and radicals.
_____________________________________________________________________
Table 2
Valences shown by common elements and radicals
Aluminum Al +3
Ammonium NH4 +1
Barium Ba +2
Calcium Ca +2
Chromium Cr +3
Cobalt Co +2
Copper Cu +1, +2
Hydrogen H +1
Iron Fe +2, +3
Lead Pb +2
Magnesium Mg +2
Mercury Hg +1, +2
Nickel Ni +2
Potassium K +1
Silver Ag +1
Sodium Na +1
Zinc Zn +2
Acetate C2H3O2 -1
Bicarbonate HCO3 -1
Bisulfate HSO4 -1
Bromide Br -1
Carbonate CO4 -2
Chlorate ClO3 -1
Chloride Cl -1
Chromate CrO4 -2
Ferricyanide Fe(CN)6 -3
Ferrocyanide Fe(CN)6 -4
Hydroxide OH -1
Hypochlorite ClO -1
Iodide I -1
Nitrate NO3 -1
Nitrite NO2 -1
Oxide O -2
Permanganate MnO4 -1
Phosphate PO4 -3
Sulfate SO4 -2
Sulfide S -2
Sulfite SO3 -2
Tartrate C4H4O6 -2
_____________________________________________________________________
Let us consider some of the elements above with positive valence values. Examination will show that in almost all cases the valence corresponds to the number of electrons in the outer shell of the element. Consider aluminum. It has a valence of +3 and Table 1 shows that aluminum has 3 electrons in its outer shell. Barium has a valence of +2 and Table 1 shows that it has 2 electrons in its outer shell. Checking shows the rule also holds for cobalt, copper, hydrogen, iron, magnesium, mercury, nickel, potassium, silver, sodium and zinc. Chromium and lead are exceptions.
Elements combine in certain ways, according to certain rules. A compound consists of two parts. One part has a positive valence and the other part has a negative valence. The sum of the positive and negative valences of the two parts must be zero in any compound. The valence of an element or radical is thus a number associated with that element or radical that helps us in constructing valid compounds. That is, it helps us in determining what combinations of elements will combine to make valid compounds and which will not.
Examples.
1. Consider the compound sodium chloride, NaCl. Here we have two atoms, one Na atom and one Cl atom. From Table 2 we note that Na has a valence of +1 and Cl has a valence of -1. The sum of the valences in the compound NaCl is thus +1 + (-1) = 0.
2. Consider the compound H2O. From Table 2 we see that H has a valence of +1 and O has a valence of -2. The compound H2O then consists of the portion H2 (two atoms of H) with a total valence of +2 and O with a valence of -2. The sum of the valences is +2 + (- 2) = 0.
3. Consider sulfuric acid, H2SO4. From Table 2 we see that H has a valence of +1 and the sulfate radical SO4 has a valence of -2. The portion of this compound with the positive valence is H2 with a valence of +2. The portion of the compound with the negative valence is the sulfate radical SO4 with a valence of -2. The sum of the valences is +2 + (- 2) = 0.
4. Consider the compound magnesium hydroxide, Mg(OH)2. From Table 2 we see that Mg has a valence of +2 and the hydroxide radical OH has a valence of -1. Magnesium hydroxide then consists of the Mg portion with the positive valence of +2 and two copies of the OH radical with a total negative valence of -2.
The above are examples of valid compounds. It is of course simple to demonstrate all kinds of combinations of elements that would not combine to produce compounds. Any two elements, both of which had positive valences, such as Al and Ca, would not combine to produce a compound. The parts of a compound thus come from two groups: 1) the group of elements or radicals with positive valences and 2) the group of elements or radicals with negative valences. The two parts of the compound must be such that the sum of the positive and negative valences is zero.
Chemical bonding. The mechanism by which two elements (or an element and radical) unite to form a compound is generally considered to be the following: the electrons in the outer shell of one atom are either transferred to another atom or shared with another atom so as to make the outer shells of both atoms as complete as possible. Thus in the case of the compound H2O two hydrogen atoms each share their single electron with an oxygen atom (whose outer shell contains 6 electrons). By this sharing, the outer shell of the oxygen atom gains two electrons and thus becomes completed with 8 electrons, providing chemical stability. With this interpretation on chemical bonding, the valence of an element then corresponds to the number of electrons gained, lost, or shared in forming a chemical bond.
Energy Levels. Corresponding to each of the seven shells is the energy level of the electrons in that shell. Electrons of the innermost shell have energy level I (also called energy level K), which is the lowest of the energy levels. Electrons of the next shell have energy level II, etc. As you go further from the nucleus successive shells have increasing energy levels, where the energy of the successive shells increase by fixed, discrete amounts. Electrons can jump from a lower to the next higher energy level if they absorb this amount of energy. Conversely, if electrons jump from a higher to a lower energy level, they give off energy, often in the form of light.
See LibreTexts Chemistry . Energy Level.
Jesus Christ and His Teachings
Way of enlightenment, wisdom, and understanding
America, a corrupt, depraved, shameless country
On integrity and the lack of it
The test of a person's Christianity is what he is
Ninety five percent of the problems that most people have come from personal foolishness
Liberalism, socialism and the modern welfare state
The desire to harm, a motivation for conduct
On Self-sufficient Country Living, Homesteading
Topically Arranged Proverbs, Precepts, Quotations. Common Sayings. Poor Richard's Almanac.
Theory on the Formation of Character
People are like radio tuners --- they pick out and listen to one wavelength and ignore the rest
Cause of Character Traits --- According to Aristotle
We are what we eat --- living under the discipline of a diet
Avoiding problems and trouble in life
Role of habit in formation of character
Personal attributes of the true Christian
What determines a person's character?
Love of God and love of virtue are closely united
Intellectual disparities among people and the power in good habits
Tools of Satan. Tactics and Tricks used by the Devil.
The Natural Way -- The Unnatural Way
Wisdom, Reason and Virtue are closely related
Knowledge is one thing, wisdom is another
My views on Christianity in America
The most important thing in life is understanding
We are all examples --- for good or for bad
Television --- spiritual poison
The Prime Mover that decides "What We Are"
Where do our outlooks, attitudes and values come from?
Sin is serious business. The punishment for it is real. Hell is real.
Self-imposed discipline and regimentation
Achieving happiness in life --- a matter of the right strategies
Self-control, self-restraint, self-discipline basic to so much in life