Website owner: James Miller
Heat engines. Carnot engine, Carnot cycle. Gasoline engine. Otto cycle. Second law of thermodynamics.
James Watt invented the steam engine in 1763. It brought about the great industrial revolution. Before the days of the steam engine men had to depend on their own muscles or on animal, wind and water power for work they wished to do. The steam engine made possible power-driven machines that could do the work of many men and brought a new era of machines, factories and automation. The modern farmer produces more with his tractor and farm equipment than hundreds of farmers could have produced before the machine age. The modern factory produces goods in vast quantities, bringing great economies and great advances in the general standard of living.
Heat engines. Today the steam engine, steam turbine, and internal combustion engine utilize the energy of fossil fuels (coal, oil and natural gas) to power the machinery of our modern society. They are examples of what are called heat engines. Heat engines can be viewed simply as devices that transform heat into mechanical energy. They do this in a two step process: 1. They first transform the fuel into heat through a combustion process. 2. They transform the heat energy into mechanical energy. In general, they perform step 2 by employing a working fluid (a gas) that takes on a quantity of heat Q, expands in a cylinder pushing a piston and doing work, thus converting part of Q into work, and then discarding the rest of Q to the surroundings (i.e. to the atmosphere).
Efficiency of heat engines. One pound of coal produces about 13,000 Btu of heat. We know that 1 Btu = 778 ft∙lb of mechanical energy so we might expect a steam engine to get 13,000×778 ft∙lb of mechanical energy from every pound of coal. In fact, a steam engine provides only from about 5% to about 30% of this value. Where does the rest of the energy go? Stack and friction losses account for only a small part of what is lost. By far the largest part appears as heat rejected in the exhaust. The efficiency of the modern gasoline engine is 15 - 20%. Again, the largest part of the heat lost is in the exhaust. That sounds like very poor economy. Can’t we devise engines that do better than that?

Carnot engine. A Carnot engine is an idealized engine operating under a Carnot cycle. The Carnot cycle is a theoretical thermodynamic cycle proposed by Sadi Carnot in 1824 and modified later by Clapeyron. It can be shown that the Carnot cycle is the most efficient cycle for converting a given amount of thermal energy into work.
In the Carnot cycle the working fluid (a gas) takes in heat at some fixed high temperature T1, converts part of it to mechanical energy in the form of work, and discards the rest to some low temperature reservoir (i.e. the surroundings, the atmosphere) at temperature T2. The amount of heat taken in is Q1, the amount discarded at temperature T2 is Q2, and the difference Q1 - Q2, is the amount converted to work.
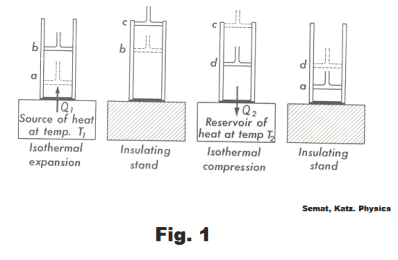
The cycle consists of four steps. The steps are detailed in Fig. 1 and 2. The cylinder in Fig. 1 is assumed to be well insulated so that no heat will pass through its walls.
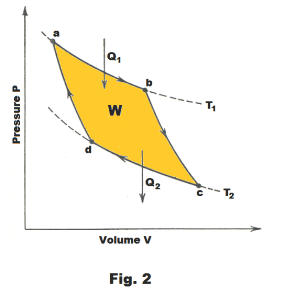
Step 1. a → b. An isothermal expansion of the gas at temperature T1 . The gas is heated and allowed to expand isothermally. It does work on its surroundings and takes in a quantity of heat Q1. See Fig. 1 and 2.
Step 2. b → c. An adiabatic expansion of the gas until the temperature drops to T2 .
Step 3. c → d. An isothermal compression of the gas at temperature T2 . Here work is done on the gas and a quantity of heat Q2 is discarded to the reservoir.
Step 4. d → a. An adiabatic compression of the gas until its temperature reaches T1. During this step compression work is done on the gas by the surroundings. On completion of this step, the gas is back at the same state as it was at Step 1 and the cycle has been completed.
It is seen that the Carnot cycle consists of two isothermal processes and two adiabatic processes. The net amount of work W done during the entire cycle is that shown enclosed by the curves, abcda, in Fig. 2 where W = Q1 - Q2.
Efficiency of a heat engine. If a heat engine takes in a quantity of heat Q1 at a high temperature T1, transforms a portion of the heat W to work, and discards the rest Q2 to a low temperature reservoir at temperature T1, its efficiency is given by the fraction of the heat taken in that is converted into work i.e.
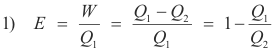
Efficiency of a Carnot engine. It can be shown that the efficiency of a Carnot engine is given by
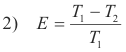
where T1 is the absolute (Kelvin) temperature at which heat is taken in by the working fluid and T2 is the absolute temperature of the reservoir to which the heat is exhausted.
No engine can be more efficient than a Carnot engine. It has been proven that no engine operating between two given temperatures can be more efficient than a Carnot engine operating between the same two temperatures. It has also been proven that all Carnot engines operating between the same two temperatures have the same efficiency, irrespective of the nature of the working substance.
The gasoline engine. A cycle of the gasoline engine consists of four strokes:
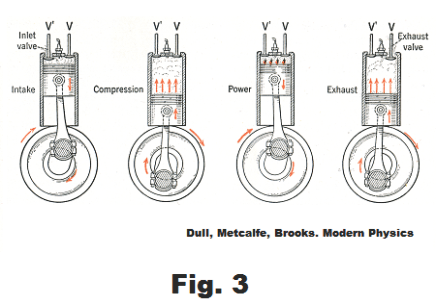
Stroke 1. Intake stroke. Starting with the piston at the top of the cylinder, the piston moves down drawing in an explosive mixture of air and gasoline vapor from the carburetor, the inlet valve being open and the exhaust valve being closed.. See Fig. 3.
Stroke 2. Compression stroke. The intake valve closes and the piston moves up, performing an approximately adiabatic compression of the air-gasoline mixture to one-seventh or one-eight its original volume.
Stroke 3. Power stroke. Just before the piston reaches the top of the compression stroke, an electric spark is produced between the points of a spark plug set in the cylinder head. The spark ignites the air-gas mixture in the cylinder and the resulting explosion produces hot gases that force the piston down in an approximately adiabatic expansion.
Stroke 4. Exhaust stroke. The exhaust valve opens and the piston moves up, forcing the exhaust gases out of the cylinder.
Otto cycle. The behavior of a gasoline engine can be approximated by an idealized cycle called the Otto cycle. An assumption is made that there is no friction, no loss of heat through the cylinder walls, and that the working fluid is air, which behaves like an ideal gas with constant heat capacities.
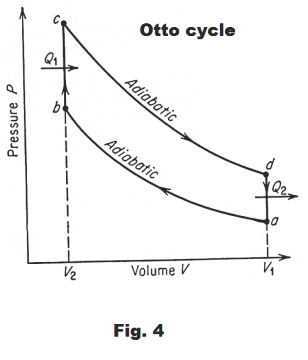
Starting at point a in Fig. 4, air at atmospheric pressure is:
1) compressed adiabatically in a cylinder to point b
2) heated at constant volume to point c
3) allowed to expand adiabatically to point d
4) cooled at constant volume to point a, after which the cycle is repeated.
Line ab corresponds to the compression stroke, bc to the explosion, cd to the power stroke, and da to the exhaust stroke of a gasoline engine.
Efficiency of the Otto cycle. The thermal efficiency of the Otto cycle is given by
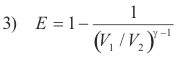
where V1 and V2 are respectively the maximum and minimum volumes of air in the cylinder and γ is the ratio of the specific heats of air, i.e. γ = cp/cv,. The ratio V1 / V2 is called the compression ratio, and is about 7 for a gasoline engine. For a compression ratio of 7 and a value of γ = 1.4, the efficiency is about 0.54. Because of friction, loss of heat through the cylinder walls, etc. the efficiency of an actual engine is much less.
Second law of thermodynamics. The second law of thermodynamics has been stated in a number of ways. Some common statements are:
1. Clausius: It is impossible to construct a machine that, while operating in a cycle, will produce no other effect than the transfer of heat from a cold body to a body of higher temperature.
2. Kelvin-Planck: It is impossible to construct an engine that, while operating in a cycle, will absorb heat from a single reservoir and produce an equivalent amount of heat.
Mechanical energy can be converted completely into heat, but heat cannot be converted completely into mechanical energy. This is an example of a one-sidedness of nature that is revealed in many ways. Heat always flows from a hotter to a cooler body and never the other way; rocks weather, iron rusts, people grow old. These are all examples of irreversible processes that take place in one direction only and express the second law of thermodynamics.
References
Dull, Metcalfe, Brooks. Modern Physics.
Sears, Zemansky. University Physics.
Schaum. College Physics.
Semat, Katz. Physics.
Kittsley. Physical Chemistry.
Warner. Thermodynamic Fundamentals.
Zemansky. Heat and Thermodynamics.
Jesus Christ and His Teachings
Way of enlightenment, wisdom, and understanding
America, a corrupt, depraved, shameless country
On integrity and the lack of it
The test of a person's Christianity is what he is
Ninety five percent of the problems that most people have come from personal foolishness
Liberalism, socialism and the modern welfare state
The desire to harm, a motivation for conduct
On Self-sufficient Country Living, Homesteading
Topically Arranged Proverbs, Precepts, Quotations. Common Sayings. Poor Richard's Almanac.
Theory on the Formation of Character
People are like radio tuners --- they pick out and listen to one wavelength and ignore the rest
Cause of Character Traits --- According to Aristotle
We are what we eat --- living under the discipline of a diet
Avoiding problems and trouble in life
Role of habit in formation of character
Personal attributes of the true Christian
What determines a person's character?
Love of God and love of virtue are closely united
Intellectual disparities among people and the power in good habits
Tools of Satan. Tactics and Tricks used by the Devil.
The Natural Way -- The Unnatural Way
Wisdom, Reason and Virtue are closely related
Knowledge is one thing, wisdom is another
My views on Christianity in America
The most important thing in life is understanding
We are all examples --- for good or for bad
Television --- spiritual poison
The Prime Mover that decides "What We Are"
Where do our outlooks, attitudes and values come from?
Sin is serious business. The punishment for it is real. Hell is real.
Self-imposed discipline and regimentation
Achieving happiness in life --- a matter of the right strategies
Self-control, self-restraint, self-discipline basic to so much in life