Website owner: James Miller
Cohesive and adhesive forces. Capillary action.

Cohesive and adhesive forces. It takes great force to pull many solids apart. An example is steel. It is obvious that there must be a very strong force of attraction between the molecules of substances like steel. The force of attraction between molecules of the same kind is known as cohesion. There can also be an attraction between molecules of different substances. For example chewing gum or various types of glue may stick to many different kinds of substances. The force of attraction between unlike molecules is called adhesion. Forces between molecules, such as those of cohesion and adhesion, are effective only over extremely short distances, distances of the order of molecular diameters. Consequently, they are called short-range forces in contradistinction to forces such as gravitational attraction which is a long-range force.
An experiment illustrating cohesive forces is the following: Take two pieces of metal, each with an accurately plane surface, and bring them together. There will be no attraction between them until they are in contact. However, once in contact, it will take a very great force to pull them apart. The experiment can be performed with two pieces of steel or two pieces of lead.
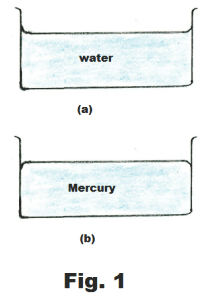
Pour some water into a glass container and the surface of the water will be level except where it contacts the glass. Where it contacts the glass it will be seen to rise above the water level for a short distance, clinging to the glass, as shown in Fig. 1(a). The water in this case is said to wet the surface of the container. Here the water molecules are more strongly attracted to the glass molecules than to themselves. That is, the force of adhesion between the water and glass molecules is greater than the force of cohesion between the water molecules.
Now pour some mercury into a glass container. The surface of the mercury will be level except where it contacts the glass, where the edges are depressed, dropping below the mercury level, as shown in Fig. 1(b). In this case the force of cohesion between mercury molecules is greater than the force of adhesion between them and the glass. If the force of cohesion is greater than the force of adhesion, the liquid will cling to itself and will tend to form droplets when placed on a smooth surface instead of spreading out to wet the surface.
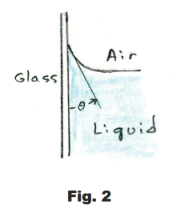
The angle θ shown in Fig. 2, between the liquid surface and the solid surface, is called the angle of contact. The angle of contact for water and glass is 0o while for
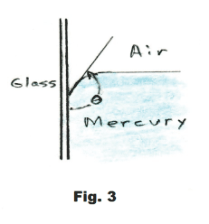
mercury and glass it is 139o. See Fig. 3.
Capillary action. If we place a tube of small diameter in a container of water the water will rise up into the tube, as shown in Fig. 4 (a), and the smaller the diameter of the tube, the higher it will rise. If we place a tube of small diameter in a container of mercury the mercury level in the tube will be depressed, as shown in Fig. 4(b), and the smaller the diameter of the tube, the more it will be depressed. This action in which a liquid rises, or is depressed, in a tube is called capillary action. Capillary action comes about as a consequence of both the adhesive forces between the liquid and the tube and the surface tension of the liquid.
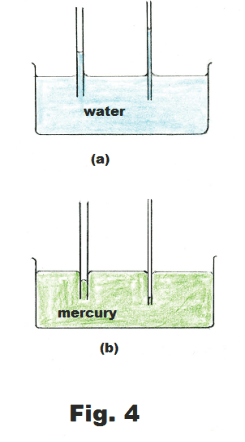
The surface of the liquid in a capillary tube is called the meniscus. If the liquid wets the tube, the meniscus is concave. If it doesn’t wet the tube, it is concave.
Experiment shows:
(1) Liquids rise in capillary tubes if they wet the tubes. If they don’t wet them they are depressed.
(2) The amount of elevation, or depression, is inversely proportional to the diameter of the tube.
(3) The amount of elevation or depression decreases as the temperature increases.
The rise of fluid in a wick and the absorption of ink by a blotter are examples of capillary action in ordinary life.
Height to which liquids rise in a capillary tube. The height h to which a liquid will rise in a capillary tube is given by the formula
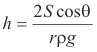
where
S = surface tension of the liquid
r = radius of the tube
ρ = density of the liquid
θ = angle of contact between the liquid and the tube
g = acceleration of gravity
_______________________________________
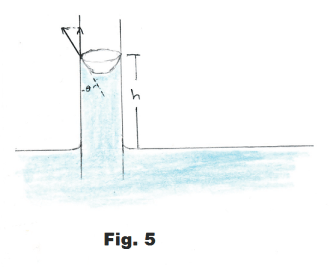
Derivation. Along the top edge of the meniscus where the liquid surface touches the wall of the tube, the tensive forces of the tensive surface film are holding up the weight of the column of liquid. See Fig. 5. At the top rim of the menscius the liquid makes an angle of θ with the tube wall. The tensive forces are tangent to the liquid surface and consequently make an angle of θ with the wall. The column of liquid is being held up by the vertical component of these tensive forces. This vertical component is given by
F = 2πrS cos θ
The weight w of the column is given by
w = ρg(πr2h)
where πr2h is the volume of the column and ρg is unit weight.
Thus
ρg(πr2h) = 2πrS cos θ
or
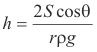
_______________________________________
Problem. A glass tube of inside diameter 0.4mm stands vertically with one end immersed in mercury. Find the depression of the mercury in the tube.
Solution. The specific gravity of mercury is 13.6, the angle of contact of mercury with glass is 139o, and the surface tension of mercury is 490 dynes/cm.
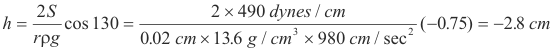
References
Sears, Zemansky. University Physics.
Semat, Katz. Physics.
Dull, Metcalfe, Brooks. Modern Physics.
Schaum. College Physics.
Jesus Christ and His Teachings
Way of enlightenment, wisdom, and understanding
America, a corrupt, depraved, shameless country
On integrity and the lack of it
The test of a person's Christianity is what he is
Ninety five percent of the problems that most people have come from personal foolishness
Liberalism, socialism and the modern welfare state
The desire to harm, a motivation for conduct
On Self-sufficient Country Living, Homesteading
Topically Arranged Proverbs, Precepts, Quotations. Common Sayings. Poor Richard's Almanac.
Theory on the Formation of Character
People are like radio tuners --- they pick out and listen to one wavelength and ignore the rest
Cause of Character Traits --- According to Aristotle
We are what we eat --- living under the discipline of a diet
Avoiding problems and trouble in life
Role of habit in formation of character
Personal attributes of the true Christian
What determines a person's character?
Love of God and love of virtue are closely united
Intellectual disparities among people and the power in good habits
Tools of Satan. Tactics and Tricks used by the Devil.
The Natural Way -- The Unnatural Way
Wisdom, Reason and Virtue are closely related
Knowledge is one thing, wisdom is another
My views on Christianity in America
The most important thing in life is understanding
We are all examples --- for good or for bad
Television --- spiritual poison
The Prime Mover that decides "What We Are"
Where do our outlooks, attitudes and values come from?
Sin is serious business. The punishment for it is real. Hell is real.
Self-imposed discipline and regimentation
Achieving happiness in life --- a matter of the right strategies
Self-control, self-restraint, self-discipline basic to so much in life