Website owner: James Miller
Prove: During an adiabatic process,
![]()
where γ = Cp / Cv .
Proof. In an adiabatic process
ΔU + W = 0
So
1) dU + pdV = 0
Now
dU = nCVdt where n is the number of moles of gas
Substituting into 1) we get,
2) nCVdt + pdV = 0
Since pV = nRT, p = nRT/V. Substituting into 2),
![]()
Dividing through by nCVT,
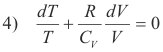
Now
5) R = CP - CV
Dividing 5) by CV gives
6) R/CV = γ - 1
Substituting 6) into 4)
![]()
Integrating gives
8) ln T + (γ - 1)ln V = ln const.
Taking antilogs
![]()
or, stated differently,
![]()
which is one of the equations we wished to prove. To derive the other we use pV = nRT and substitute T = pV/nR into 9) to get
![]()
or
![]()
which can be rewritten as
![]()