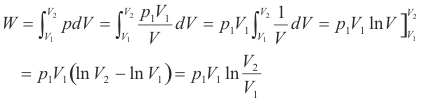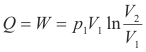
Website owner: James Miller
Prove: When a mass m of gas expands isothermally against a pressure, the change in internal energy is zero and the heat added is given by
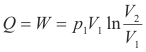
Proof. Boyle’s law holds, so p1V1 = p2V2, or equivalently, p1V1 = pV. Thus